Dedicated to the Cargo Cults of Biology Science, Biotechnology and the Pharmaceutical Industry. "So we really ought to look into theories that don't work, and science that isn't science" Richard Feynman, Cargo Cult Science, From a Caltech commencement address given in 1974
Search This Blog
Thursday, November 30, 2006
FDA Recommends Celebrex
WASHINGTON (AFX) - A federal advisory panel recommended that Pfizer Inc's Celebrex should be approved to treat rheumatoid arthritis in children, despite worries about long-term safety of the drug, the Wall Street Journal reported. The US Food and Drug Administration's committee of outside experts voted 15-1 in favour of using Celebrex to treat children with the condition, but said that adverse events must be closely monitored in the long term, the paper said.
Would you give your kid a cox-2 inhibitor?
Wednesday, November 29, 2006
Eight to Ten Weeks to Live
Here's the crazy part. Most of the deaths have been attributed to disease progression — meaning the advancement of the patient's cancer. The study's patients have advanced lung cancer, with a life expectancy of 8 to 10 weeks.
How much life can be added to a population of people who have 8 to 10 weeks to live. Another statistical gamble has been lost. It could have easily gone the other way. Xyotax has been shown to do nothing. They kept changing the beer and pizza diet and they got burned. But guess what. This isn't the end. Beer and Pizza diets never end!
The company expects to revise the study when it resumes, to focus on women with normal estrogen levels. CTI is claiming earlier Xyotax studies had found a better effect in such women. So if you've got 8 to 10 weeks to live due to lung cancer AND you've got normal estrogen levels, get yourself signed up for this one.
Seattle Biotech Dustbowl
 laboratory workers to compete for a handful of jobs. This is not very well known. Like Boca Raton and Charlotte NC, we want to be known as a biotech hot spot. We want to be the next Boston or San Diego. The Cargo Cult Scientist wants the same thing because he needs a job. We understand that advertising the plight of the skilled labor here will not serve anyones purpose. But no one reads this blog, so we are free to discuss what is happening.
laboratory workers to compete for a handful of jobs. This is not very well known. Like Boca Raton and Charlotte NC, we want to be known as a biotech hot spot. We want to be the next Boston or San Diego. The Cargo Cult Scientist wants the same thing because he needs a job. We understand that advertising the plight of the skilled labor here will not serve anyones purpose. But no one reads this blog, so we are free to discuss what is happening.Profits
Nov. 2--Senior executives at Icos are in line to receive cash payments worth a combined $67.8 million for selling the company to Eli Lilly, according to a filing Wednesday with the Securities and Exchange Commission.
At the top of the list is Paul Clark, 59, Icos chairman, chief executive and president, who will receive a "golden parachute" worth $23.2 million in severance pay, cashed-out stock options, restricted stock awards and other bonuses for retention and closing the deal.
Others cashing out big include Executive Vice President Gary Wilcox ($8.5 million), Chief Financial Officer Michael Stein ($7.1 million) and Chief Medical Officer David Goodkin ($5.9 million).
Rank-and-file employees of the Bothell-based company will not fare nearly as well.
I've also talked about a major investor, Healthcors plans to vote against the sale of ICOS. But I wanted to talk about another lucky devil, Greg Weaver of SIRNA. SIRNA, you will remember was bought out by Merck for 1.1 billion bucks. Mr. Weaver had left Nastech in 2005 "to pursue other opportunities." He landed the CFO gig at SIRNA six months before the Merck take-over. Damn the luck, he was out on his ass again. How much did he leave with? Four million dollars!
Now here's the hard part of being an executive. His replacement at Nastech came from ICOS. Doh! Phil Ranker left ICOS after being passed over for a promotion. Now he's making good money but nothing makes an executive feel better than a multi-million dollar payday. What did Greg Weaver do right? He was fired from Nastech. What did Phil Ranker do wrong? He quit ICOS.
The Hwang Paper
In order to correct for their embarrassing inability to tell shit from shinola, the journal appointed a panel to review the review process that led to the incident. The editor of Science, Donald Kennedy, said that the journal accepts the panels major findings. What the panel decided was that this whole thing could have been prevented by extra review procedures. Is anyone seeing a pattern yet?
These panels are populated by reviewers. What good would adding more people and more reviews do? It's like trying to clean off a muddy windshield with a newspaper. No matter how much you scrub you just end up smearing the mud from place to place. More reviews creates more chatter but it doesn't have the power of going into the lab and repeating an experiment. What if this latest panel on the Hwang case would have made the recommendation to have key experiments verified by a non-partial contract research organization? Perhaps such a recommedation would diminish the perceived authority of a scientific panel.
The Science panel said that a risk assessment method should be developed to flag high-visibility papers for further review. Also, authors should specify their individual contributions to a paper, a reform aimed at Hwang's stratagem of allowing another researcher, Dr. Gerald Schatten of the U. of Pittsburgh, to be a lead author of one of the reports even though Schatten had done none of the experiments. The Cargo Cult Scientist would like to point out the no respectable scientist in this age ever does an experiment. They may run a lab where the experiments are done but they are as close to the work as any other co-author whose name is of value to the paper. As for the risk assessment method to be developed, we see another panel being formed in the future.
A scientific paper was once a thing of great beauty. A scientist was once a person who observed nature first hand and offered up explanations that made the world take notice. The ease with which Dr. Hwang hoodwinked this major journal sent out a message. We're doing something wrong. We have a system where highly ambitious people are told that the only way to accomplish there ambitions is to publish papers on how great they are at conducting research. Negative results are not very welcome. The best way to get published is to tell the editors what they want to hear. If, for example, RNAi is popular with a journal you must validate the concept by reportinig how well it knocked down your gene of interest. Failures are just not welcome. How many papers were published last year about RNAi experiments that failed? How many people just couldn't clone a stem cell? Dr. Hwang said he could and he was published. Hmm.
We have a dream here at the CCS. Just as the computer revolutionalized the way offices are ran throughout the world, we believe they can help medical science. A computer is a cold and impersonable entity. It doesn't care of you need to tell your boss that the ELISA assay worked out as planned. It can only report the data it receives. I'll end with a simple example of how this could work.
An ELISA can be developed to the point where all a technician needs to do is add the constituents and read the plate. The plate has a code indicating that the ELISA is, for example, one of three. If one of the ELISAs needs to be thrown out it must be justisfied. The cubicle scientist must understand the system and interpret the data based on the data. NO BEER AND PIZZA DATA HANDLING. If a scientist is forced to design experiment to the point where this will work, the scientist will become a better researcher. Reviewers will have to really understand the designs they are reviewing. Will this ever become a reality? We here at the Cargo Cult Scientist are forming a panel to discuss this very question.
Tuesday, November 28, 2006
The Bridge

Although I haven't seen it, I am very interested in the concept. You know that over 40 people a year jump off the bridge. You point a camera to capture the last moments of a persons life then you go find out about that person. I suppose you could just look up suicides in the city coroners
 office but the bridge is special. People come from all over the country, to jump to their deaths from the bridge. Why such a lonely pilgrimage. Is there something they are trying to gain by associating their death with the bridge? Do they feel a kinship to the others who have taken the same route? Does it give them strength? There is something to learn here.
office but the bridge is special. People come from all over the country, to jump to their deaths from the bridge. Why such a lonely pilgrimage. Is there something they are trying to gain by associating their death with the bridge? Do they feel a kinship to the others who have taken the same route? Does it give them strength? There is something to learn here.We are interested in gaining understanding into how the human mind works. Mostly however, we are interested in studying how people study things. The makers of "The Bridge" pointed a camera at the bridge and waited. That was the start. What if medical researchers had such a start? We know certain people are predisposed to cancers. Why can't we watch their cells? Why can't we desing methods to identify cells who are ready to jump off "The Bridge"? One way of telling if a cell has lost it's will to live is through aneuploidy. If the number of chromosomes is not complete, then you may be looking at the origin of cancer. It's just a thought. It is unfortunate that most scientists these days observe nature second hand from lab techs.
All of this brings me to an observation I made while watching two college professors discuss whether or not the situation in Iraq could be called a civil war or not. Both men were arrogant old white scholars whose expertise was political science and historical conflicts. Of course they were not willing to call the situation in Iraq a civil war. A reporter who has been there off and on for three years had a different take on the situation. "If this isn't a civil war, I'd hate to be here when one finally breaks out." The reporter laid out some criteria and explained how all of this was taking place. But professors who works in offices thousands of miles away from the sights and sounds of war do not see it the same way. Why believe the guy who isn't there? There is a struggle involving life and death taking place. The best way to understand it is to observe what is happening. Ask the people what they saw. Find out why the thing happened. We're not going to learn anything about this mess listening to politicians and college professors. They talk pretty, but that is what they have learned through observations. They mimic the tones and repeat what they've heard. But they have never been to the bridges where stories begin. Where are the ones who have sat by and watched a story begin, end or just take place for awhile? Why listen to people who are never there?
FDA Questions Pfizers Beer and Pizza Diet

Monday, November 27, 2006
So Much for the $800 Million Pill
Nov. 27, 2006
Biotechnology Development Costs Top $1.2 Billion Per Product
The average cost of developing a new therapeutic biotechnology product is more than $1.2 billion, including the costs of drugs that fail in testing and the time associated with bringing these products to market, according to a new study by the Tufts Center for the Study of Drug Development.
Beer and Pizza Diet

A fat man goes to his doctor for a new diet to help him lose weight. The doctor advises him to eat pizza and drink beer for every meal. One month later the fat man returns to the doctor weighing an additional ten pounds. "What kind of beer did you drink? Coors? Well why'd ya do that. You need to drink a microbrew."
You can imagine the endless game the doctor is trying to engage in. One o
 f the things most white lab coat lab techs know is the Beer and Pizza diet. You are given an experiment to do. Most often the instructions are verbal. Sometimes they are sketched out on a chem wipe. Never, never, never (in R&D) are they fully written out. Material and methods sections are written only after the data that was desired has been obtained. It may have taken 10 tries but on the tenth try the data worked out in the cubicle scientists favor.
f the things most white lab coat lab techs know is the Beer and Pizza diet. You are given an experiment to do. Most often the instructions are verbal. Sometimes they are sketched out on a chem wipe. Never, never, never (in R&D) are they fully written out. Material and methods sections are written only after the data that was desired has been obtained. It may have taken 10 tries but on the tenth try the data worked out in the cubicle scientists favor.Is it wrong then to talk only about the tenth experiment? With a Beer and Pizza diet you can easily explain away the first 9. In experiment 1 the lab tech used the wrong brand of sodium chloride. In experiment 2 the cells were used at the wrong confluency. When you get the data that you want, the Beer and Pizza questions needn't be asked. If the data fits, you did the right thing. If it doesn't, you did something wrong.
I once worked with a supervisor who was charged with screening antibodies. When the antibodies failed the western blot test, the questions began. One lab tech was told to repeat a western blot because she had only boiled the protein samples 4 minutes instead of 5. She rolled her eyes and repeated the western. Same result. The supervisor threw up her arms stating that if this tech was foolish enough to boil protein samples for 4 minutes (instead of 5) that there could be a million other things she was doing wrong. One day a good antibody showed up and passed the western blot test. We boiled the samples 4 and 5 minutes and asked the supervisor to point out which were which. She explained that good antibodies sometimes work so well that you cannot see the subtle difference. "Could you show us an example of one that can show the subtle difference." She didn't respond.
Moving higher up the big pharma food chain we have the latest success of Pfizer and Celebrex. Celebrex was the first of a class of new painkillers, called cox-2 inhibitors, approved in December 1998, and it is the last one to remain on the market. Two years after Vioxx was taken off the market and Merck has spent millions defending themselves and their own Beer and Pizza approach to clinical trials, Pfizer is asking the FDA to expand the use of Celebrex. They want to give it to kids as young as 2 who have arthritis. FDA advisers will meet Wednesday to consider the company's request. Why would one cox-2 inhibitor be the cause of so many lawsuits yet another still be on the market and expanding its applications?
Sunday, November 26, 2006
Build It and They Will Come, North Carolina Style
The University of North Carolina plans to spend 29 million dollars a year on billionaire David Murdock’s biotech real estate development venture in Kannapolis. That money is on top of the hundreds of millions in local tax subsidies to help build the research campus.
Currently there are not many biotech companies lining up for lab space at the site. So far, only two companies to be exact. Still, to prepare for the coming blizzard of employment, another major component of the Kannapolis project is a training center run by the N.C. Community College system to prepare workers for what Murdock contends will be thousands of new biotech jobs. Let's add up the reality. We have two companies and a promise of thousands of jobs. But wait, there's more. Clyde Higgs, Castle & Cooke’s vice president of business development, said he has received nearly 60 business plans from companies seeking venture capital for business in medical devices, information technology and drug development. Now we have two companies, a promise of thousands of jobs and 60 business plans that need money.
Do biotech business plans generate money are do they take money? In other words, are local governments investing in biotech or are biotech executives investing in local governments? The Cargo Cult Scientist has posted his observations on the emptiness all around the south Lake Union where Paul Allen is trying to create a Northwestern biotech hub. San Diego is crumbling. When will we see a successful biotech hub? When will the planes land? We've built more than a few Cargo Cult Airports. Each one has kept businessmen and government officials gainfully employed. But they keep coming back asking for more money. When will biotech become an asset and not a liability. If the people of North Carolina think biotech will be an immediate asset, they are wrong. It will be a liability. The question isn't about how much they are paying now. The question they need to ask themselves is how much they will be willing to spend in the next ten to 20 years.
Wednesday, November 22, 2006
Giving Thanks
Alright, I am feeling sorry for myself. I have applied for everything you can imagine, not just biotech. I had a phone interview with a company that washes yachts. Each morning you get in your own little boat and hit the various marinas around the area to wash yachts and give tune-ups. I was ready to hang up my career and do some real work for a change. They told me they were worried I'd just leave as soon as I got a biotech offer. The pay started at 11 dollars an hour. Still, I was disappointed by this rejection. I could just see myself heading out in darkness and fog at 7 a.m. I wanted to get out there and smell the Puget Sound and look up at the cars on the I-5 as they crawled along to their miserable jobs. It still hurts. I even wrote them back asking for reconsideration. Nothing.
I believed that biotech needed scientists who knew all about DNA, proteins and how to study them. I'm quite sure the HR lady, who was so smug as I left the building at my last job, had a degree in liberal arts. She once told us how she was going to see to it that "this company becomes a huge success." I'm thinking, why don't you see to it that MetLife pays my dental bill so I don't get sued! We had a little pissing contest before I left. She was trying to show me how much more she knew about the business. "Do you have any experience with in vivo animal cells?" "Do you mean mammalian cells inside an animal?" We just stared at each other. Prior to hearing "in vivo animal cells" I had no reason to dislike her. On my way out the door, with my box of belongings we smiled at each other under a cloud of mutual resentment.
I made a mistake. I entered a field where PhDs are made into supervisors instead of talented and experienced researchers. I've had to explain DNA chromatograms to senior level scientists who list molecular biology as part of their expertise. I've had to explain the development of an ELISA assay to the director of QA. His development procedures usually began by purchasing the assay from Amersham then changing a buffer or two. I've spent 6 months trying to get difficult concepts into the heads of cubicled science people only to start all over when the lawyer joined in on the meetings. Now it's all over. The industry only wants younger kids to come in and do the lab work. They're cheaper. Anyone can do the work.
I'll give thanks when and if they let this crash test dummy out of this car that is speeding towards the wall. I continue to play the game of hide and seek a living wage. Until then I am thankful to the New Belgium Brewery in Fort Collins Colorado for making Fat Tire beer. I may not make any money but I can't go back to Pabst Blue Ribbon. I haven't sunk that far yet.
Tuesday, November 21, 2006
A New Cargo Cult
PALO ALTO, Calif.--(BUSINESS WIRE)--Intradigm Corporation, a privately held biotechnology drug development company focused on the discovery and development of RNA interference (RNAi) therapeutics for the treatment of diseases with unmet medical needs, has achieved several significant corporate developments. These developments encompass strengthening the senior management team, completing a $16 million Series A financing, and establishing new research and drug development facilities in Palo Alto, CA.
Intradigm was formed in 2000 to develop proprietary nucleic acid delivery technology. The fundamental platform of Intradigm's technology is a ligand-targeted nanoparticle system that is capable of systemic delivery of multiple RNAi molecules targeting different genes.
"The discovery that RNAi can silence gene expression is a major scientific breakthrough, and has led to numerous developments towards the use of this technology as a treatment modality," said Dr. Mohammad Azab, CEO of Intradigm. "However, the translation of that discovery to a therapeutic drug is hampered by lack of effective delivery systems. Intradigm is developing such a system and we intend to aggressively pursue the realization of the therapeutic promise of RNAi using our technology."
New research and drug development facilities in Palo Alto, CA
From the Cargo Cult Scientist:
The company started in 2000, before RNAi had been discovered. When they say, "nucleic acid delivery technology," they are side stepping the fact that they were a gene therapy company. The long suffering gene therepy scientists were given a reprieve with RNAi. They simply morphed into RNAi specialists. Gene therapy, RNAi, what's the difference? It's all nucleic acid magic.
Welcome to the Caro Cults of this blog Intradigm. I await your contributions to this fascinating area of biotechnology. We'll be keeping track of your progress.
Monday, November 20, 2006
RNAi Mice
Artemis is the first company to have developed a robust methodology for the controlled induction of shRNA based gene knock down (6) in adult mice. This inducible system permits the down-regulation of a selected target gene to be turned on and off thereby more closely modeling the dosing of a pharmacologic inhibitor. The generation time for such a mouse model at Artemis is only four months.
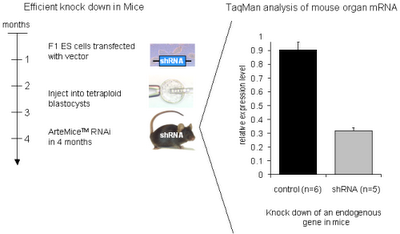 First they clone in the siRNA sequence that will knock out an endogenous gene product. They are using Taqman to measure mRNA levels. Let's assume that this is the accepted method of measuring RNAi knock down. Wouldn't a better control be to not induce the RNAi in one mouse and induce it in another. That way you are comparing the effects of RNAi and not two genetically different mice. According to Artemis:
First they clone in the siRNA sequence that will knock out an endogenous gene product. They are using Taqman to measure mRNA levels. Let's assume that this is the accepted method of measuring RNAi knock down. Wouldn't a better control be to not induce the RNAi in one mouse and induce it in another. That way you are comparing the effects of RNAi and not two genetically different mice. According to Artemis:Using a reporter system, we have demonstrated that in our system constitutive RNAi knock down of over 80% can be achieved in almost all tissues of the body. Of what protein?
This knock down has been shown to be stable over at least 25 weeks and is inheritable into the next generation. At 80% KO strength?
Development of a system to efficiently produce RNAi knock down mice within 4 months. Why does it take less time than making other genetically altered mice?
We have shown that RNAi-based constitutive knock down of endogenous genes can reproduce the phenotype of the corresponding conventional gene KO. Such as???
Tuesday, November 14, 2006
Navy Photo

I took this picture when I was in the Navy. The boss sent me out on a Saturday. A submarine was toting a mini sub around. That's all I knew then and all I know now. I'm not into submarines. I was into photography back then.
A lot of my photos were saved but I can't find them in the DOD files. The point of this post is that we all go out do work each day. How much lasts? This is all I can find on the internet of my five years as a Navy photographer. Mostly I took pictures of re-enlistment and retirement ceremonies.
I can remember eating that dry yellow cake with lard and powdered sugar frosting at the ceremonies. I can even recall a couple of speeches that the retiring Navy guys made. The most memorable one was from a man who grabbed the American flag they gave him and held it high above his head. "This is what it was all about," he said with tears in his eyes. About a month later I was taking pictures of all the stuff had stolen while in the Navy. His whole apartment was purchased by the American people.
That's it. Just a Navy picture and a Navy story.
N Rays and Me
 gs me to the subject of N-Rays. My zero readers will be familiar with the N-Rays era of scientific progress. In 1903 French scientist René-Prosper Blondlot discovered N-Rays. The Germans had discovered X-Rays so many scientists at this time were looking for undiscovered regions on the electromagnetic spectrum that could make them famous. The problem with N-Rays was that they don't exist. For a short while they did however, in the minds of physical scientists in the early 1900s. One day an American scientist named Robert W. Wood travelled to France to have Blondlot show him N-Rays first hand. The experiment required the use of a prism. When the lights went low Dr. Wood slipped the prism into his pocket. The experimenters still "observed" their N-Rays. Dr. Wood reported his own experiment and that was the end of N-Rays.
gs me to the subject of N-Rays. My zero readers will be familiar with the N-Rays era of scientific progress. In 1903 French scientist René-Prosper Blondlot discovered N-Rays. The Germans had discovered X-Rays so many scientists at this time were looking for undiscovered regions on the electromagnetic spectrum that could make them famous. The problem with N-Rays was that they don't exist. For a short while they did however, in the minds of physical scientists in the early 1900s. One day an American scientist named Robert W. Wood travelled to France to have Blondlot show him N-Rays first hand. The experiment required the use of a prism. When the lights went low Dr. Wood slipped the prism into his pocket. The experimenters still "observed" their N-Rays. Dr. Wood reported his own experiment and that was the end of N-Rays.Thursday, November 09, 2006
RNAi For Dummies
I happen to think that when someone takes the time to discuss the details we should listen. The CEO of Nastech took the time today in the form of a webinar. At the end there was a Q and A session. Here is what I learned. It is the third great leap in biotechnology. Number one was cloning, two was monoclonal antibodies and number three is RNAi. Small molecule drugs bring in over 175 billion per year from over 1000 products. Monoclonals bring in 40 billion form 200 products. RNAi brings in zero dollars from zero products. However, RNAi is potentially trillions of times more potent than monoclonal antibodies. The major issue facing biotechnology is the delivery of the RNA to the RISC complex inside of the cell. Nastech has a delivery peptide (PN73) that modulates tight junctions and could be used to deliver RNAi.
The webinar was a humorous example of a businessman talking to his investors. The businessman seemed to have a need to educate his investors, so he presented what I call RNAi for Dummies. After the one hour lecture it was Q and A time. I was curious like a child. How does it get inside the body? How long does it last and where does it go when it's done working? What does PN73 do to a tight junction protein? But here is what the class wanted to know.
1) Will Nastech be receiving more NIH money?
2) What else does Nastech do?
3) What are the timelines for filing INDs?
4) Is the board open to an acquisition like Mercks acquisition of Sirna.
5) When will you file for an IND?
If you want to be successful in biotechnology you have to be prepared to ask the tough question. When do we start making our money?
Friday, November 03, 2006
ICOS Executives Cash In
But wait just a minute! A statement from HealthCor Management LP, a major ICOS investor, sent a shot across the bow of the board of directors. "We cannot imagine how the compensation commuttee could have possibly justified the audacious handout or how the board of directors failed to stop it. We remain confused as to to how the board of directors of ICOS can so generously calculate the value of management performance yet simultaneously so conservatively undervalue the assets of the company that is being managed by this same group." Ewwww!!! Tales of biotech backstabbing!
Things are heating up in Bothell Washington. "We're waiting to hear whether Lilly will keep any presence in Bothell", Icos spokeswoman Lacy Fitzpatrick said this week. Well I got some sour news for ya, Lacy. You're going to get laid off with the rest of them. After HealthCor piped up on the BS executive payouts, some experts wonder if the investors will reject the merger offer. Not likely. They're getting a good deal. Cialis is all ICOS had to offer. Lilly is taking it. Bothellonians are now free to wander.
As this Titanic biotech ship continues it's ascent to the upward position prior to its thunder plunge, the Cargo Cult Scientist will keep his zero readers abreast of the ICOS demise. There are over 600 human beings who earned their livings off of the company. A handful are in like Flynn. 180 sales people are out on the streets. I hope they still have their good looks! That leaves 500 and some odd humans left wondering when and how it all ends. We'll be waiting. First things first however, the execs got paid.
Thursday, November 02, 2006
Novartis Goes to China

From Biospace:
Swiss pharmaceutical company Novartis AG is to invest $100 million in building a biomedical research and development center in Shanghai, China. Research and development activities at the site will initially focus on addressing urgent medical needs in China and Asia, particularly infectious causes of cancer endemic to the region, Novartis said. "The level of scientific expertise in China is rising rapidly. At the same time, the healthcare needs of the Chinese are growing, primarily the result of urbanization, lifestyle changes and associated chronic diseases," said Dr Daniel Vasella, chairman and CEO of Novartis.
From the Cargo Cult Scientist:
There are 1.6 billion people living in China. They've been getting by in the Chinese way for a long time. We have a 100 million dollar culture clash coming. Chinese medicine meets western corporate medicine. What I wouldn't give to be a Chinese speaking fly on the wall at some of these meetings.

